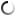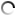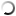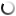 loading
loading
featuresBuilding a better mouseA Yale geneticist and a Chinese lab are creating the Amazon.com of medical research animals. Margot Sanger-Katz ’02 is senior staff editor of the Yale Alumni Magazine. 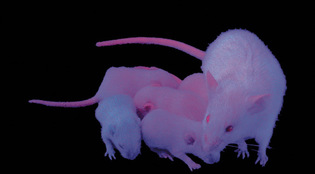 Tian Xu ’90PhDTian Xu's mutant mice glow pink under ultraviolet light, thanks to a custom-built piece of mobile DNA. View full imageMice like to bury marbles. If you give a laboratory mouse a handful of marbles, it will often bury a few into the bedding in its cage. But one particular mouse in Yale genetics professor Tian Xu’s lab will bury marbles all day long. That mouse is missing a gene. Having seen it at work, Xu and his colleagues now think the gene may be related to behavioral problems like obsessive-compulsive disorder. Another mouse of Xu’s appears to have the mouse equivalent of male-pattern baldness. It grows lovely white fur all over its body, but its head is bare. Studying the gene this mouse is missing could help scientists understand what causes some men to lose the hair on their heads. Tian Xu ’90PhD, who has developed an innovative system for easily producing mice missing single genes, is raising thousands of mutant mice in a laboratory complex in China—mice that don’t grow properly, mice that have kidney disease, mice with neurological problems, mice that lack sex appeal—in hopes that researchers around the world can use those mice to better understand human development and disease. His goal is a “functional map” of the mouse genome, linking every gene in the mouse to its function in the species. Such a tool has never been created for a mammal, and it could have major implications for human medicine. Humans and mice share about 99 percent of their genes, making mice, which are small, inexpensive to raise, and (relatively) short-lived, an ideal animal model for studying human disorders and testing possible treatments. Biologists have been studying mice for decades, using them to learn about physical and mental development, cancer, and heart disease, among other subjects. The scientists who developed the first system for disabling single genes in mice won the Nobel Prize in Medicine in 2007. Over the last two decades, scientists who needed mice missing specific genes have used gene splicing and other methods to engineer them for lab work. Texas A&M Health Science Center professor Richard Finnell, for instance, studies spina bifida, a birth defect that can lead to lifelong paralysis. In 2006, he developed a mouse with damage to a gene called FKBP8, which controls the development of the spinal column. (He calls the mouse “Stacey.”) He has used its descendants ever since in his research on the environmental factors that contribute to the birth defect. All told, scientists have so far succeeded in knocking out about a fifth of the 24,000 genes that have been mapped in the mouse genome. Xu’s breakthrough, developed at Fudan University in Shanghai, where he has an adjunct faculty appointment, was in creating a simple and inexpensive system for disabling different genes quickly and efficiently—a sort of assembly line for mutants. In the first 18 months of his project, he’d already reached the 5,000-gene mark that took previous scientists 20 years. Instead of the painstaking work of splicing coded bits into mouse DNA, placing the manipulated DNA into embryonic stem cells, and then implanting those cells into pregnant mouse wombs—a process that can take an individual lab as long as a year and cost more than $50,000—Xu can breed a mouse with a disabled gene simply by mating two specially prepared mice. One of the mice has a piece of genetic material called a transposon, which can jump within the genome of the reproductive cells, inserting itself at random to disable an existing gene. The other makes an enzyme that activates the jumping gene. Breed the two together, and each of their offspring will be born with one inactive gene, a different gene in each. The process doesn’t require molecular biologists, just skilled technicians who can care for the mice and run basic tests to determine which gene is missing. Transposons are common in plants and insects, and scientists have used a similar technology to create mutants in insect species. But before Xu, no one had been able to find a transposon that would work efficiently in mammals. And, in a clever twist, he has figured out a way to color-code the mice so that technicians can tell instantly whether a mouse is normal or a mutant: the mutant mice carry a gene that makes them glow pink under an ultraviolet lamp. The mutants are bred in a lab in China large enough to house 150,000 mice at a time. Overseen by about 150 Chinese lab technicians and scientists—and U.S. researchers via webcam—the lab is breeding its way through the mouse genome. Xu expects to disable nearly every mouse gene in a few years, at a fraction of the cost required to complete the project the traditional way. “There’s a significant advantage to doing this stuff in China, because it’s just so cheap,” says Colin Fletcher, program manager for the National Institutes of Health’s (NIH) Mouse Knockout Project, which has funded part of Xu’s research. The project is also funding other scientists to knock out 8,500 genes in mouse stem cells using more-conventional technologies; its goal is to make a complete “library” of knockout mouse stem cells available to researchers. In China, Fletcher says, Xu’s work sees cost savings in building construction and lab equipment, but particularly in labor. A technician who would earn a salary of more than $30,000 in the United States can be hired for about $4,500 in China.
|
|